THERMOCHEMICAL REACTIONS
Thermochemistry is the science of analyzing
molecular reactions to determine if they are spontaneous, energy absorbing or
releasing, and to predict the product mole ratios and rates. Chemical
reactions, like most other processes, tend to follow the path of free energy
minimization or entropy maximization. This principle forms the mathematical basis
for the analytical approach. Mixing of different chemical species, such as
water and wine, changes both. Except for such things as water and oil—which
don't mix—substances that do mix, will not separate spontaneously. Separation
requires work—if it can be done at all. Chemical reactions produce different
molecules or species. The species preceding the reaction are called reactants
and those following or ensuing from the reaction are called products.
Free energy accounts for the quantity
we commonly associate with energy and also the quality. The quality
of energy may be thought of as it's capacity to perform useful work and
is related to organization or molecular arrangement. The free
energy is equal to the common energy, e, less the entropic term, or e-Ts, where
T is the temperature and s is the specific entropy. For a closed
system (one that doesn't exchange mass with its surroundings), we have the Helmholtz
free energy: a=u-Ts, where u is the specific internal energy. For an open
system (one that does exchange mass with its surroundings), we have the Gibbs
free energy: g=h-Ts, where h is the specific enthalpy.
How will a reaction proceed? It will seek
the path resulting in the least free energy. How then are thermochemical
reactions solved? By finding the outcome that results in the minimum free
energy. Will just any such outcome suffice? No, because there are other
constraints, for example, conservation of energy or the 1st Law of
Thermodynamics. Minimum free energy is actually a statement of the 2nd Law of
Thermodynamics. So, we seek the solution that results in the least free energy
and also the conservation of energy. Therefore, solving this mathematical problem
is called: nonlinear constrained minimization. In short, we refer to
this as: The Gibbs Problem. This is what CREST does for you. Reactions
can be relatively simple, as in this first illustration (combustion of octane):
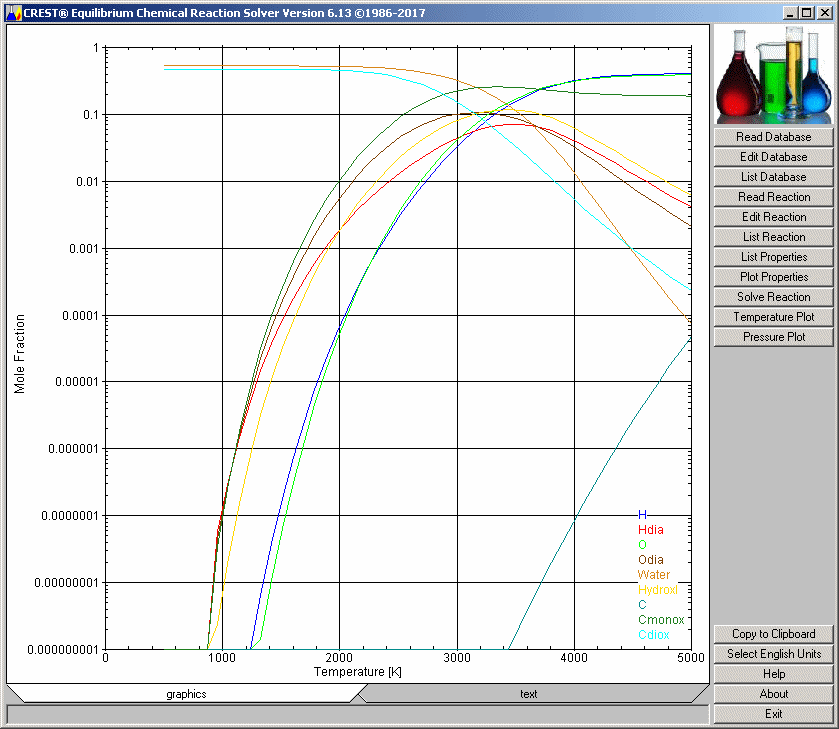
Combustion of Octane with
Oxygen
or
quite complex, as illustrated in this next figure (combustion of landfill gas consisting of many substances):
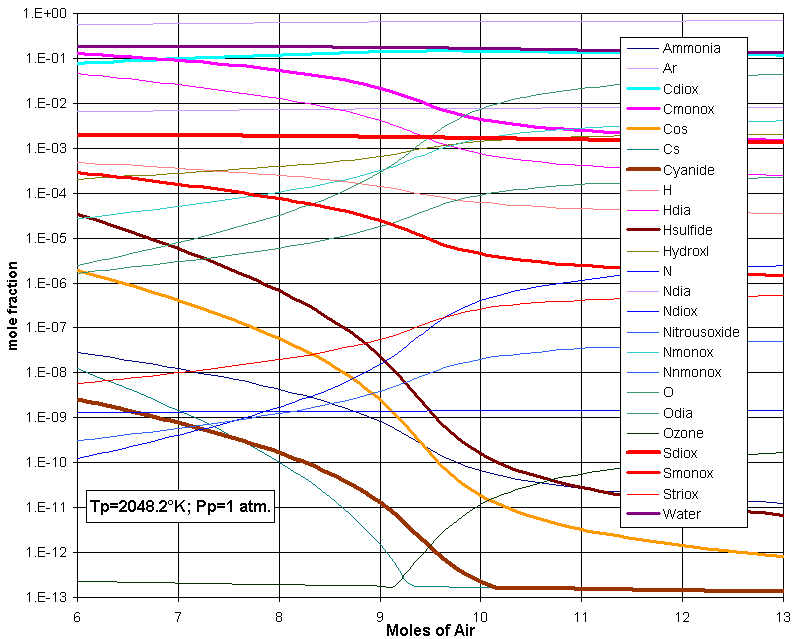
Combustion of Landfill Gas
This next figure shows a "perfect storm" of chemical confusion at an air/fuel ratio of 9.6, exactly what you don't want to happen...

Combustion of Natural Gas with Ammonia
This next figure arises from the introduction of limestone in the combustion of coal in order to capture the sulfur:

Combustion of Coal with Limestone
This next figure illustrates the dissolution of transite (asbestos fiber cement board):
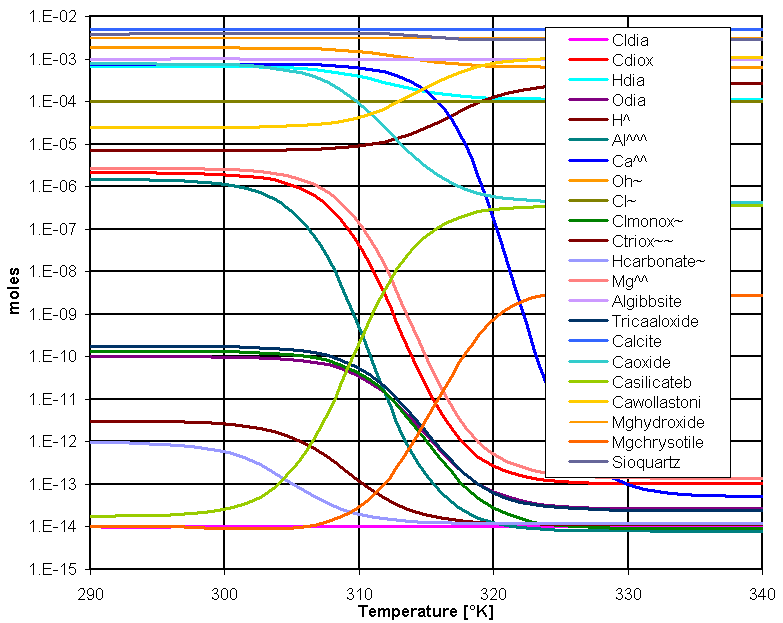
CREST
will vary some parameters (such as temperature, pressure, and moles of one
component) and solve for a range of conditions or you can launch it from
another program and feed it varying inputs, as illustrated below:
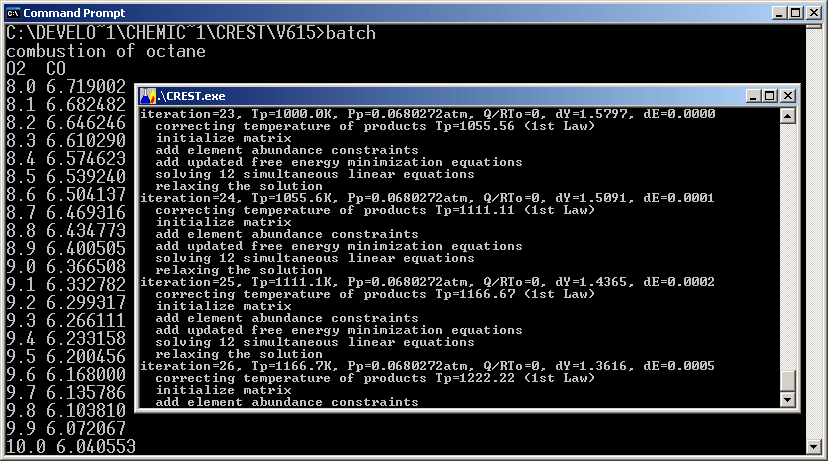
Running CREST in Batch Mode
Something
unique about CREST is the ability to handle non-ideal behavior. The
original approach (called the RAND algorithm) presumed that the
reactants and products were ideal gases. This facilitated calculation
of the specific energy, entropy, and free energy plus led to a stable
solution (Newton iteration and the Method of Steepest Descent). For
many reactions (such as combustion at atmospheric pressure) this is
adequate; however, for others it is not. An example of when the
assumption of ideal behavior is not adequate is the disolution of
transite above, where liquids, aqueous ions, and dissolved gases are
involved. The same equations must be solved but the standard techniques
simply don't work. It took me years to solve this problem, which began
with being stranded in Chicago's O'Hare Airport for three days during a
blizzard and took a completely different project plus a programming
error to stumble across a robust solution. The result is now free to
all!
This is all described in my book, Thermochemical Reactions, https://www.amazon.com/dp/B07SW9LWTS
The
latest version of CREST can be found on the software page: software